About Us
Who are we?
NOAH is the trade association representing the UK animal health industry. We support our members’ businesses and ensure the sustainability of our sector by helping shape the regulatory, political and social climate.
Find out more – watch our video ‘Supporting the future for animal health’
What do we do?
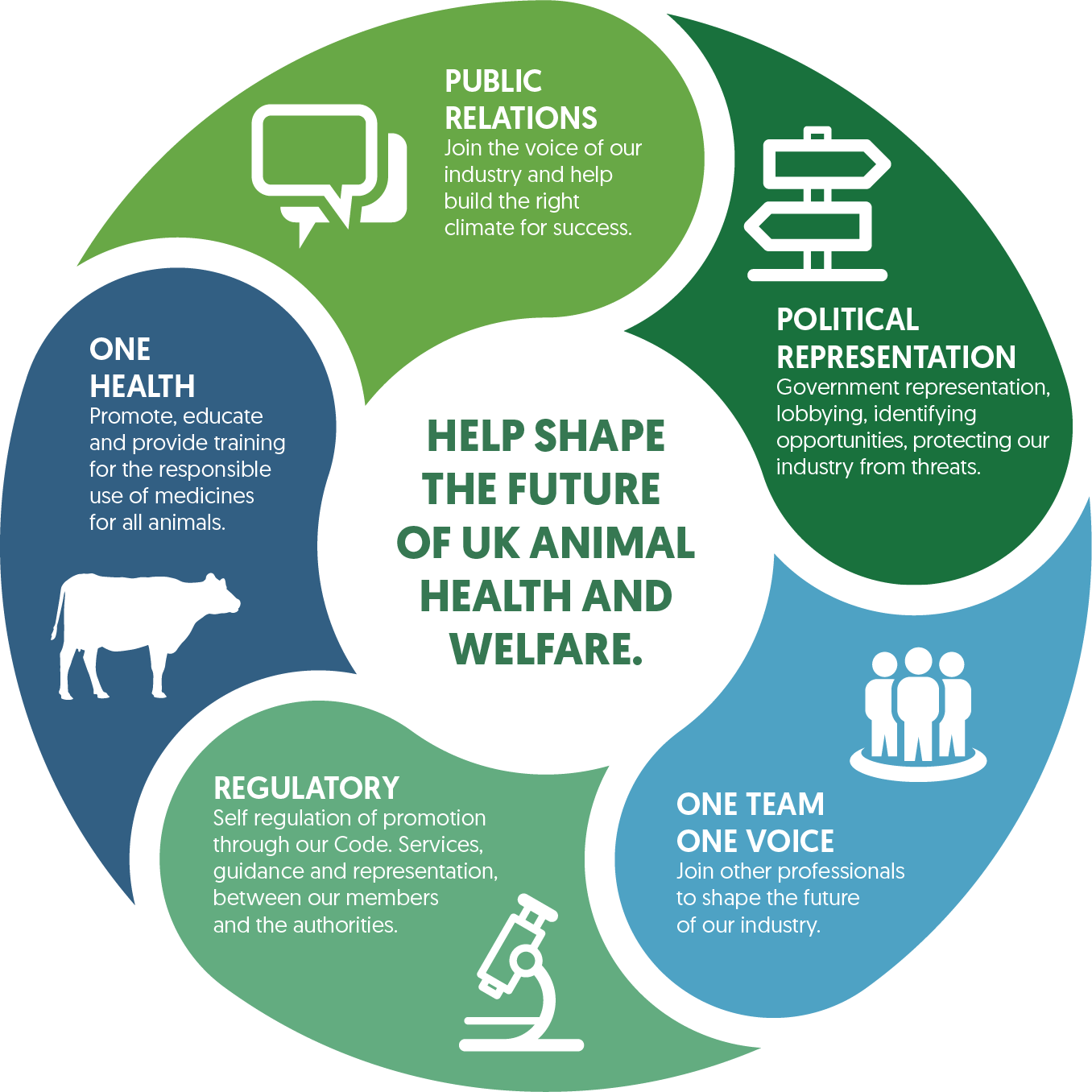
We foster innovation and promote responsible use of our members’ products. Our vision is to be at the forefront of UK animal health and welfare, championing licensed medicines and solutions for all animals.
Our focus
- Support business continuity and opportunity for all medicines and animal health solutions, ensuring end users’ access to licensed medicines
- Promote a robust regulatory environment, to ensure a high standard of UK animal health and welfare and meet the needs of our members
- Ensure appropriate access and responsible use of medicines for all animals, whilst protecting animal, human and environmental health (One Health)
- Support a political / social climate where our members’ businesses can be successful
- Work collaboratively with members to define policy priorities; communicate effectively to ensure NOAH remains the trusted expert partner on all UK veterinary medicine and animal health issues, providing horizon scanning and thought leadership
Find out more about membership
Email us at: noah@noah.co.uk
Our Mission
NOAH represents the UK animal health industry. We promote the benefits of licensed medicines and solutions for the health and welfare of all animals.
NOAH also:
- Promotes and defends the responsible manufacture, promotion, sale, distribution, handling and use of animal medicines.
- Acts as a consultative body to the industry, Government, the media and the general public.
- Represents the views and interests of its members.
- Communicates the benefits of animal medicines to animal health and welfare, and sustainability.
The animal health industry plays a key part in:
- Farm and companion animal welfare
- Safe food for consumers
- Farm sustainability and resilience
- Healthier pets supporting human wellbeing
- Environmental protection
- Scientific innovation
Our strategy

Our environmental policy
NOAH’s latest Environmental Policy can be found here.

