Industry Facts and Figures
NOAH member companies account for a total of around £745 million in annual UK sales of authorised veterinary medicines, at ex-manufacturers’ prices, net of all discounts. This figure is based largely on companies who participate in the NOAH/CEESA independent sales survey, plus those who provide their sales data separately. NOAH membership now represents in excess of 97% of the UK animal medicine market.



Medicines to keep animals healthy by preventing disease account for the majority of sales. Some of the main groups of medicines used to treat and prevent disease in farm and companion animals include:
- Vaccines: prevent diseases caused by certain viral, bacterial or parasitic infections by stimulating the body’s own immune system
- Ectoparasiticides: these medicines prevent and treat infestation by parasites that live on the outside of the animal, for example mites, ticks, fleas, lice and flies
- Endoparasiticides: these medicines prevent and treat infestations by parasites that invade the body, such as worms, flukes or coccidial protozoa
- Endectocides: prevent and treat infestation of both endo- and ectoparasites.
- Antimicrobials (antibiotics): treat bacterial infection
- Medicines to treat reproductive problems
- Anti-inflammatories (pain killers)
- Other therapeutic medicines (including anaesthetics and other products acting on the nervous system; medicines for digestive problems; cardio pulmonary therapy; geriatric medicines and medicines to treat or prevent specific dietary deficiencies, as well as a number of other niche medicines).

Preventing and protecting animals against harmful or zoonotic disease, such as through vaccination, contributes positively towards ‘One Health’ (an integrated, unifying approach that aims to sustainably balance and optimise the health of people, animals and ecosystems), including the responsible use of antimicrobials as well as to sustainable livestock farming, companion animal welfare, environmental management, and food security.
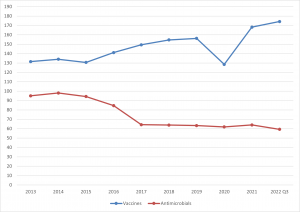
Trends of sales of vaccines and antimicrobials to end Q3 2022
Sales by species

![]()




In 1986, when NOAH was formed, around 70% of animal medicines were used in farm livestock. Now over 60% animal medicine sales, by value, are for companion animal use.
While farm animal numbers have declined, British farmers are still investing in the health of their livestock, particularly in the vaccine sector. The species split is particularly indicative of exciting new product developments in the companion animal sector – Britain’s pets have every opportunity to live long and healthy lives.
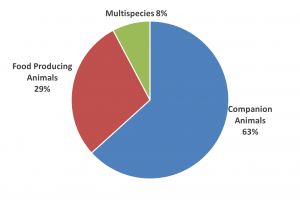
UK market by species, 12 months to end Q3 2022
Sales by legal category
The following abbreviations in the chart relate to the four different legal categories under which authorised veterinary medicines can be sold in the UK under the Veterinary Medicines Regulations (VMR).
- POM-V: Prescription Only Medicine – Veterinarian. A veterinary medicinal product which may only be supplied to the client once it has been prescribed by a veterinary surgeon following a clinical assessment of an animal, or a group of animals, under the veterinary surgeon’s care.
- POM-VPS: Prescription-Only Medicine – Veterinarian, Pharmacist, Suitably Qualified Person (SQP). A medicine for food-producing animals (including horses), to be prescribed by any Registered Qualified Person (RQP means a veterinarian, pharmacist or an appropriately qualified SQP). A clinical assessment of the animal(s) is not required when prescribing this category of veterinary medicine and the animal does not have to be seen by the prescriber. However sufficient information about the animal and the way it is kept must be known to the prescriber in order to prescribe and supply appropriately.
- NFA-VPS: Non-Food Animal Medicine – Veterinarian, Pharmacist, Suitably Qualified Person. A medicine for companion animals may be supplied by any RQP (a veterinarian, pharmacist or Suitably Qualified Person) provided the requirements for supply are met. These medicines do not require a prescription.
- AVM-GSL: Authorised veterinary medicine – general sales list. There are no legal restrictions in the VMR for the retail supply of veterinary medicines as AVM-GSL, but a responsible approach to the supply of these medicines is still expected.
All medicines for food-producing animals need to be prescribed, according to their legal category (so are POM-V or POM-VPS). All antibiotics, whether for food producing or companion animals, are classified as POM-V and are only available under prescription from a veterinary surgeon.
In addition two other product types are included in the NOAH Compendium of Data Sheets:
- Exemptions for Small Pet Animals (ESPA): Certain medicines can be placed on the market without a marketing authorisation (MA), subject to certain conditions. This exemption applies only to veterinary medicines labelled exclusively for use in one or more of the following animals that are not intended for human consumption: aquarium animals (including fish kept in closed water systems), cage birds (meaning birds kept in cages or aviaries), homing pigeons (meaning pigeons kept for racing or exhibition), terrarium animals (meaning reptiles, amphibians and arthropods kept in tanks and cages – including animals free-living in domestic gardens), small rodents (meaning domestic mammals of the order rodentia), ferrets and rabbits.
- Specified Feed Additives Feed additives authorised under Regulation EU 1831/2003 belonging to the functional groups; coccidiostats, histomonostats and certain other zootechnical additives.(NB these are not included on the chart)
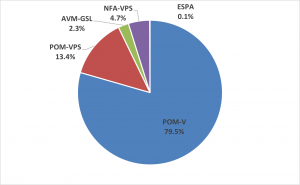
UK market by licence category 12 months to end Q3 2022