Back to Topics
Veterinary Medicines: Regulation and Environmental Risk Assessment
Introduction- regulation of veterinary medicines in the UK
In the UK, the national competent authority and independent regulator is the Veterinary Medicines Directorate (VMD). The VMD are responsible for authorising, as well as monitoring a veterinary medicinal product’s safety, quality and efficacy following market authorisation (also known as pharmacovigilance). The VMD also develop, update, and enforce all legislation concerning veterinary medicines, regulating from the start point of manufacturing through to administration, with the legislative requirements and framework set out in the UK Veterinary Medicines Regulations (VMRs) 2013. These laws provide all legislative requirements concerning the manufacture, classification, supply, marketing, and use of veterinary medicines.
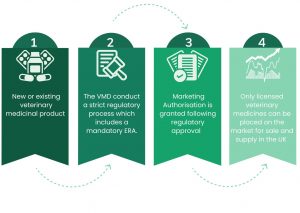
All the authorised veterinary medicines available in the UK for animals must undergo a strict regulatory approval process, before they can be granted a Marketing Authorisation (MA) (the product licence) and before they can be placed on the market for sale and supply. The regulatory system ensures that all veterinary medicinal products that are marketed in the UK have undergone an independent scientific assessment for quality, efficacy and safety, including safety to the environment.
Only authorised veterinary medicines can legally be used in the UK. The enforcement division of the VMD proactively takes action, which can include prosecution, against any illegal marketing and use of unauthorised products in relation to breaches of the VMRs.
A downloadable version of this document is available in our resources here.
Environmental Risk Assessment of veterinary medicines: an ongoing review
For veterinary medicines to be granted a marketing authorisation in the UK, they must all undergo an environmental risk assessment (ERA) based on their expected use (VMD,2022). The ERA is an essential evaluation, ensuring new or current veterinary medicinal products do not pose a wider threat to human, animal, or environmental health.
After veterinary medicines are placed on the market, their safety remains under ongoing review with regulatory oversight. From the start of 2021, following the UK’s exit from the European Union, this ongoing regulatory oversight is completely within the remit and control of the VMD: prior to that the UK was part of the EU’S regulatory review process. Since the European referral procedure was developed as part of the European Veterinary Medicines Directive (2001/82) in 2001, 19 referrals have been triggered as a result of environmental concerns, with the outcome from two of the 19 referrals concluding that the overall benefit/risk balance was negative and resulting in the withdrawal of the affected products from the market 2. This example demonstrates the robustness of the regulatory system, and that the safety of veterinary medicines is kept under ongoing review with regulatory decisions being adapted depending on what the scientific evidence indicates is appropriate.
The ERA: how the benefit/risk evaluation is conducted
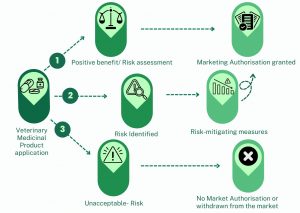
The ERA is based on a benefit/ risk evaluation that provides guidance to help assess the environmental impact of veterinary medicines and to help implement risk minimising measures. The main purpose is:
- To identify potential environmental hazards and risks
- To identify the need for specific risk-mitigation measures (if appropriate)
- Ensure appropriate labelling advice and instructions are in place for end users and prescribers.
Only when a veterinary medicine is known to have a positive benefit to risk assessment will the product be authorised to enter the market. In cases where a potential risk is identified, the ERA will proceed to identify specific risk-mitigating measures, which will be clearly stated on the product label. If the product poses unacceptable risk, which is not outweighed by its therapeutic benefits, the authorisation of the product will be denied or withdrawn from the market. The summary outcome of any veterinary medicine’s ERA is available publicly, either via the Veterinary Medicines Directorate website or via the European Medicines Agency, depending on where the product was initially authorised.
The ERA evaluates the use of the medicine, the physico-chemical, ecotoxicological and fate properties of its active substance. The scope of the ERA does not cover environmental contamination caused by the manufacturing process, transport, or storage of the medicinal product; these matters are regulated by the authorities under separate regulatory processes.
The ERA is tier based and comprises two phases (Phase I and Phase II).
Phase I: Environmental Impact Assessment
The Phase I assessment is mandatory for all veterinary medicines. It determines the environmental exposure of the veterinary medicine and if a further ecotoxicological assessment is required (Phase II).
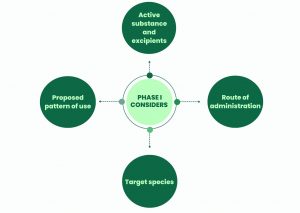
The Phase I guideline consists of a decision tree that determines if the veterinary medicinal product achieves the criteria for the risk-assessment to end at Phase I, or if it requires a higher tier assessment (Phase II).
The majority of pharmaceutical veterinary medicinal products (> 95%) are considered to have a limited environmental release, and their risk assessment ends in the lowest tier (Phase I).2
Phase II: Ecotoxicological assessment
If a veterinary medicinal product’s environmental exposure is not considered to be negligible after a Phase I assessment, a Phase II ecotoxicological assessment will be required. Generally, this is the case for veterinary medicines used in aquaculture, intensively reared terrestrial animals, or pasture animals.
The ecotoxicological assessment is tier based and is designed around the risk quotient, that indicates the probability of adverse effects on the environment. The risk quotient is the ratio between the predicted environmental contamination of the medicinal product at its highest concentration in any environment which no adverse effects are expected (predicted no-effect concentration).
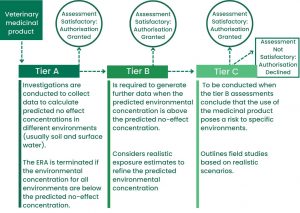
If the ERA determines the use of veterinary medicine will create risk for the environment, sometimes the company and regulatory authorities can agree mitigation measures, for example special precautions and warnings about correct use of the products, to address and reduce any risks to an acceptable level. If mitigation measures are unable to achieve this, the veterinary medicine will not receive a marketing authorisation.
Conclusion
In the UK the VMD is responsible for authorising and regulating veterinary medicines. It is mandatory for all veterinary medicines to undergo an Environmental Risk Assessment (ERA) before receiving a Marketing Authorisation. Even after a product is authorised, the VMD have the regulatory powers and responsibility to consider new evidence as it emerges and to change the indications for use and warnings or even to fully withdraw a product from the market if it negatively impacts the environment and the benefit/risk balance of the product is considered negative.
The robust regulatory system that exists for veterinary medicines in the UK ensures that prescribers and end users of veterinary medicines can be confident that the products they use are safe for the animal being treated, for the person administering the medicine and for the environment.
References and further reading
- European Medicines Agency. 2022. Environmental risk assessment of veterinary medicines – European Medicines Agency. [online] Available at: <https://www.ema.europa.eu/en/veterinary-regulatory/marketing-authorisation/environmental-risk-assessment-veterinary-medicines>
- Fabrega, J. and Carapeto, R., 2020. Regulatory review of the environmental risk assessment of veterinary medicinal products in the European Union, with particular focus on the centralised authorisation procedure. Environmental Sciences Europe, 32(1).
- NOAH (National Office of Animal Health). Controls on veterinary medicines – NOAH (National Office of Animal Health). [online] Available at: <https://www.noah.co.uk/topics/regulation/controls-on-veterinary-medicines/>
- VMD, 2022. Marketing authorisations for veterinary medicines. [online] Available at: <https://www.gov.uk/guidance/marketing-authorisations-for-veterinary-medicines>.